Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Miyashita, Sunao; Toyoshima, Atsushi; Oe, Kazuhiro*; Sato, Tetsuya; Asai, Masato; Tsukada, Kazuaki; Nagame, Yuichiro; Sch del, M.; Kaneya, Yusuke; Haba, Hiromitsu*; et al.
del, M.; Kaneya, Yusuke; Haba, Hiromitsu*; et al.
no journal, ,
The extraction behavior of hexavalent and reduced Mo and W were investigated when aqueous phase was 0.1 M HCl/0.9 M LiCl solution. The D value of Mo was changed from 10 to 1 when applied potential was near 0 to -0.2 V. On the other hand, the D value of W was not changed at all applied potentials in this experimental condition. Those results indicated that Mo was reduced by FEC, and the extraction behavior of reduced Mo was different from hexavalent Mo. In the case of W, W was not reduced or the D values of reduced W was same as hexavalent W.
Toyoshima, Atsushi; Li, Z.*; Asai, Masato; Sato, Tetsuya; Kikuchi, Takahiro*; Kaneya, Yusuke*; Kitatsuji, Yoshihiro; Tsukada, Kazuaki; Nagame, Yuichiro; Sch del, M.; et al.
del, M.; et al.
no journal, ,
The redox behavior of mendelevium (Md) was studied with flow electrolytic chromatography. The  Md isotope produced in the
Md isotope produced in the  Cm(
Cm(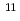 B, 4n)
B, 4n) Md reaction at the JAEA tandem accelerator was used in the electrolytic reduction experiment with the flow electrolytic column apparatus in 0.1 M HCl. It was clearly observed that, applying appropriate potentials on the chromatography column, the most stable Md
Md reaction at the JAEA tandem accelerator was used in the electrolytic reduction experiment with the flow electrolytic column apparatus in 0.1 M HCl. It was clearly observed that, applying appropriate potentials on the chromatography column, the most stable Md is reduced to Md
is reduced to Md . The redox potential of the Md
. The redox potential of the Md = Md
= Md + e
+ e couple was determined to be -0.16
couple was determined to be -0.16  0.05 V vs. a normal hydrogen electrode.
0.05 V vs. a normal hydrogen electrode.
 -spectroscopic studies
-spectroscopic studiesAsai, Masato
no journal, ,
Current status of spectroscopic studies for superheavy elements are reviewed. In particular, some of our experimental results on  -
- coincidence spectroscopy and
coincidence spectroscopy and  fine-structure spectroscopy for odd-mass No, Lr, and Rf isotopes using a gas-jet transport technique are introduced, and what are revealed in the
fine-structure spectroscopy for odd-mass No, Lr, and Rf isotopes using a gas-jet transport technique are introduced, and what are revealed in the  -spectroscopic studies is presented.
-spectroscopic studies is presented.
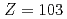 ) on surface ionization process
) on surface ionization processSato, Tetsuya; Asai, Masato; Sato, Nozomi; Tsukada, Kazuaki; Toyoshima, Atsushi; Oe, Kazuhiro*; Miyashita, Sunao; Sch del, M.; Kaneya, Yusuke; Nagame, Yuichiro; et al.
del, M.; Kaneya, Yusuke; Nagame, Yuichiro; et al.
no journal, ,
In order to determine the first ionization potential of lawrencium (Lr, 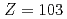 ), we have developed a surface ionization type ion-source coupled to a He/CdI
), we have developed a surface ionization type ion-source coupled to a He/CdI gas-jet transport system for an Isotope Separator On-Line (ISOL) of the JAEA tandem accelerator experimental facility. We measured ionization efficiencies of short-lived Lr isotope and various short-lived lanthanide isotopes with our present system. We successfully ionized and mass-separated
gas-jet transport system for an Isotope Separator On-Line (ISOL) of the JAEA tandem accelerator experimental facility. We measured ionization efficiencies of short-lived Lr isotope and various short-lived lanthanide isotopes with our present system. We successfully ionized and mass-separated  Lr ions for the first time and evaluated the first ionization potential of Lr based on a correlation between an effective ionization potential and ionization efficiencies of the lanthanide isotopes.
Lr ions for the first time and evaluated the first ionization potential of Lr based on a correlation between an effective ionization potential and ionization efficiencies of the lanthanide isotopes.
Tsukada, Kazuaki; Toyoshima, Atsushi; Asai, Masato; Kasamatsu, Yoshitaka*; Li, J.*; Ishii, Yasuo; Haba, Hiromitsu*; Sato, Tetsuya; Nagame, Yuichiro; Sch del, M.
del, M.
no journal, ,
We will report studies of ion-exchange chromatographic behavior of element 104, rutherfordium (Rf), and element 105, dubnium (Db), in JAEA. The experiments based on an atom-at-a-time scale have been performed using an automated rapid ion-exchange separation apparatuses, AIDA and AIDA-II. We have found interesting information for the complex formations of Rf with chloride, nitrate, sulfate, and fluoride ions and Db with fluoride ions in aqueous solutions.